For the first part of my process of growing pre-designed sculptural bone, here's what I'll be working on this week at SymbioticA: 1) Making bone soup and; 2) decellularization.
The 'bone soup' is the very particular chemical formula required for specific bone cells to grow once they have been either
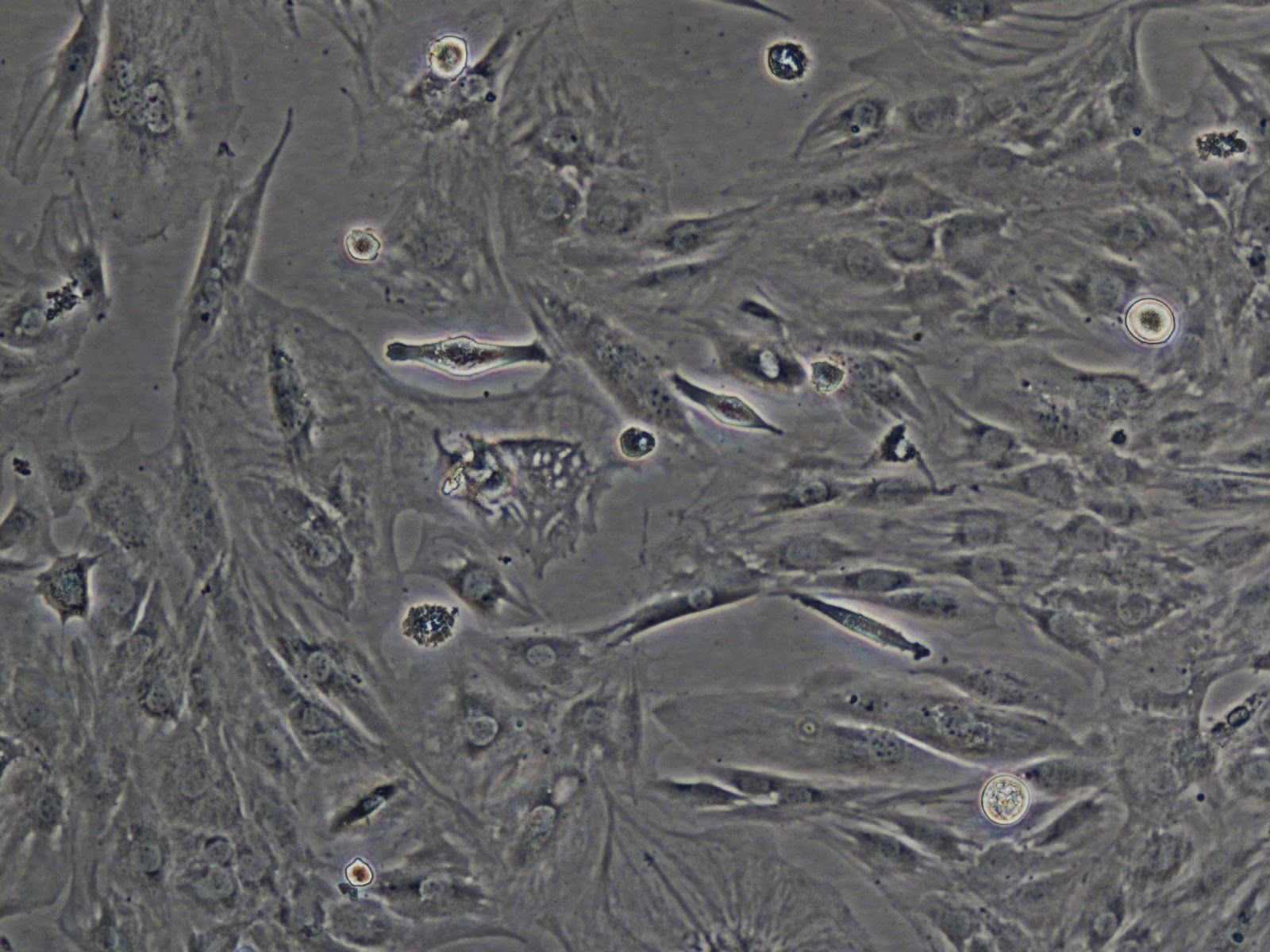
purchased as cell lines, or isolated from a bone sample and/or differentiated into the types I want to work with. There are different types of bone cells, but I want to work with osteoblasts and osteoclasts, the cells that both chew up (destroy) and build, or rebuild bone matter in a symbiotic process.
I've transcribed the Bone Soup Recipe further below. It is not lost on me that my lab work, making bone soup, is a fun metaphor for what happens in my kitchen at home. The fact is, DIYBio (do-it-yourself biology) is a growing BioArt movement that sees kitchen labs happening all over the world. At MY home, I just make bone broths to eat for good health. Anyway, I enjoy the metaphor and the witchiness of making bone soup in the lab.
I'll be visiting a butcher either later today or tomorrow to purchase some tissue and organs for decellularizing, and perhaps later in the week, going back for some freshest of fresh bone, once the bone soup is ready. Fresh, unfrozen bone contains living bone cells for a short period of time, and they can be extracted for lab use. The organs and bones will all be animal (nonhuman) samples for now but there is the potential for extracting and working with human bone cells here at UWA after an ethics approval has been obtained. That is currently in process via a UWA biologist who is interested in doing this, and who I may have the chance to work with. I may also have a chance to visit and observe the morgue and all of the human cadaver specimens this week, or next. The morgue is just downstairs from the SymbioticA office. There is an atmosphere of deep respect for everyone who has donated their bodies to science, here at the School of Anatomy & Human Biology.

Decellularization is the process of removing all cells from the tissue extracellular matrix, or the elastic-like webbing that forms the underlying structure of all animal tissue (collagen). I'm decellularizing muscle tissue (like steak) and small organs (like a chicken heart) to be left with collagen strands that I can then manipulate by hand under a microscope, into small textile-based sculptural forms. The heart, I will just use to make a ghost organ because they are beautiful, white and see thru structures in the shape of the original organ. The new scaffolding I create will then be enculturated with my new bone cell cultures, meaning I'm essentially making a collagen trellis for my new bone cell babies to grow up on and take the shape of. I also caught wind of a biologist who is decellularizing bone, Dr Mary Lee, and I meet with her tomorrow. If she'll share her protocols with me, I can do some bone decellularizing, too, meaning create transparent bone webs,
I think. I'll know more about that process after we meet tomorrow. It may be that after I decellularize bone, I can manipulate that matrix more effectively than muscle tissue matrix - I don't know yet, but it's a hunch.
Another artist/biologist I was able to meet with today is named Guy Ben-Ary, who is in charge of the microscopes and imaging in the labs. With his help, I'll be able to shoot HD video of my live cell cultures in action, squirming and growing, as well as capture high res images or videos of my hands working under the microscope to manipulate the collagen (once I have it). Also, this week I'll be watching a PhD student do the process of bone cell differentiation, which means coaxing cells to become one type or another depending on the chemicals you bathe them in. This is a process I will also have to do in my bone tissue culture work, so this is my chance to watch and learn. Very exciting. I'll post another update on the weekend to share what I was able to achieve with the bone soup and the decellularization.
Here is some technical information regarding the process (below), including the bone soup recipe:
FIRST,
Understanding basic molecular units:
1 Mole [Molar] = 10[27] = No. of molecules/L
mM = milliMolar
µM = microMolar
1/1000 = 1mM = 1 x 10[-3] M
1/1,000,000 = 1µM = 1 x 10[-6] M
MW (molecular weight) or
FM (formula weight)
MW Ascorbic acid (cell culture grade) = 176.12g
So, 176.12g/L = 1M
NOW,
Bone Soup Recipe (Osteoblast): --->
Osteoclasts will require separate recipe
- 500mL α-DMEM
- 10% FBS
- 1% Penicillin-Streptomycin
- 0.2mM Ascorbic acid
- 1mM ß GP (for mineralization only)
- 10nM dexamethasone
Coat dish with media and FBS, put in incubator for 20 mins and wash.
For later - FIX methods:
- 4% Paraphormaldehyde
- Methanol 20 mins in -20
In order to get 0.2 (1/5) mM Ascorbic acid, make STOCK SOLUTION:
> if 176.12g/L = 1M
> then 1.76G/10mL = 1M
> and 0.00176g/10mL = 1mM (1/1000 M)
> so, 1/5 of 1mM =
0.2mM
*Thank you to Dr Stuart Hodgetts, Assoc. Professor, Spinal Cord Repair Laboratory, Experimental and Regenerative Neurosciences at the School of Anatomy and Human Biology, University of Western Australia for helping me with these calculations and for future assistance in providing chemicals, equipment and guidance for making the bone soup. Also, for lending me these two books:
Carleton, H. M.
Histological Technique for Normal and Pathological Tissues and the Identification of Parasites (3rd ed.)
. Oxford University Press: London. 1957.
Hall, Brian K.
Developmental and Cellular Skeletal Biology. Academic Press: NY. 1978.
*Thank you to Ionat Zurr, Assistant Professor, SymbioticA, School of Anatomy and Human Biology for the Bone Soup recipe (taken from Visconti Lab, Dept of Veterinary and Animal Sciences, UMassAmherst, Boston, Massachusetts) and for introductions to helpful biologists.
Wish me luck with my bone soup making! It has to be inconceivably precise.






.JPG)
.JPG)
.JPG)






.JPG)
.JPG)








.JPG)







